Syneika One
Neuronavigation for Transcranial Magnetic Stimulation
Developed in collaboration with doctors and nurses, Syneika One is the only fully integrated neuronavigator offering you precision and ease of use in your daily work.
The Syneika One neuronavigation equipment for TMS offers gesture assistance and image guidance. It assists users of repetitive Transcranial Magnetic Stimulation (also known as rTMS), which is used in neurology, psychiatry and rehabilitation.
To do this, it uses the patient's previously acquired MRI imaging data. It thus gives the manipulator information on the location of the stimulation coil in relation to the target of the cerebral cortex that he wishes to stimulate, and thus helps with the precision and reproducibility of the sessions.
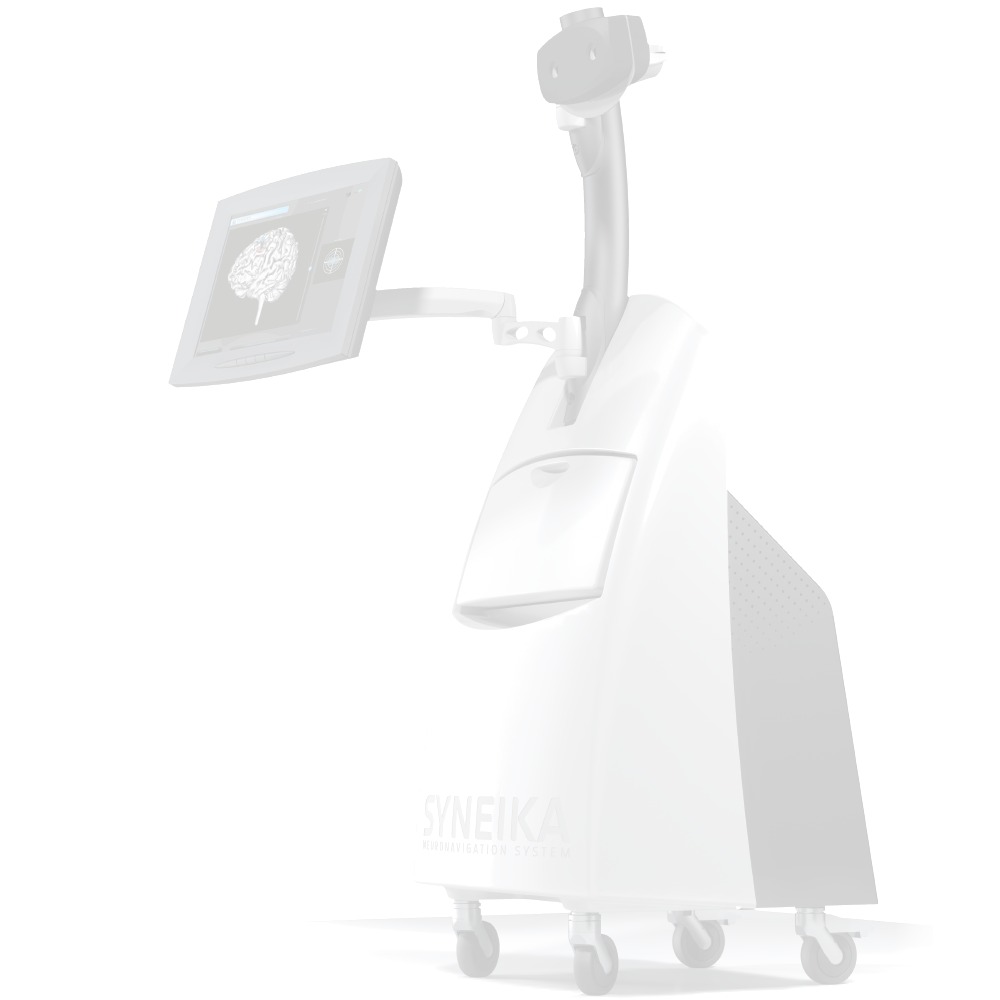
Syneika One neuronavigation for TMS allows each patient benefit from an individualized therapy
-
Productivity
Syneika One neuronavigation for TMS aims to make repetitive Transcranial Magnetic Stimulation therapy accessible to any care team. It has been designed to fit the time constraints of an intensive therapeutic activity. To this end, powerful algorithms process the data in minutes, registration of the patient is guided step-by step through an intuitive and efficient workflow.
Syneika One neuronavigation forTMS allows performing individualized therapy with an unequaled simplicity.
-
Focus
Based on the MRI to fit exactly each patient's individual anatomy, Syneika One neuronavigation for TMS guides the positioning of the TMS coil. Thus it provides optimum accuracy. This is fundamental to both the quality of your treatments and for the reliability of your clinical studies.
Simple and intuitive, Syneika One neuronavigation is handled easily by each member of the healthcare team.
-
Immediate Care
Syneika One users do not rely on any third-party expertise for handling data and start an accurate neuronavigated Transcranial Magnetic Stimulation session. Whoever the operator is, Syneika One neuronavigation for rTMS guarantees you an unparalleled localization reproducibility. This simplified usage allows each operator to fully concentrate on their patient.
SyneikaONE neuronavigation is a Medical Device, a regulated health care product that wears, in accordance with this regulation, the CE marking.
In the United States, federal law regulates the sale of medical devices through the Food and Drug Administration (FDA). These regulations are designed to guarantee the safety and efficacy of products. As the Syneika One neuronavigator is not authorised by the FDA, the use of Syneika One for positioning the transcranial magnetic stimulation (TMS, rTMS) coil is considered experimental in the United States.
Regulations in other countries: For regulations concerning the Syneika One neuronavigator outside the European Union or the United States, please contact us.
Update : 2022-05-28.
